Other
Fibrosis and Epicardial Adipose Tissue Impair Left Atrial Function in Patients with Atrial Fibrillation
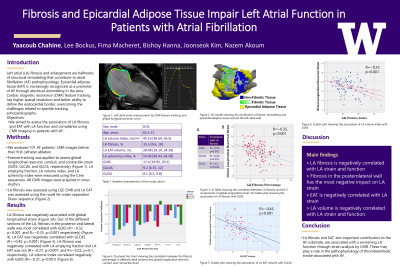
Purpose: Left atrial (LA) fibrosis and enlargement are hallmarks of structural remodeling that contribute to atrial fibrillation (AF) pathophysiology. Epicardial adipose tissue (EAT) is increasingly recognized as a promoter of AF through electrical remodeling in the atria. Cardiac magnetic resonance (CMR) imaging is the gold standard for evaluating LA volumes and function, and feature tracking in CMR has been confirmed as a feasible and reproducible technique for assessing LA function through strain. We aimed to assess the association of LA fibrosis and EAT with LA function and compliance using CMR imaging in patients with AF.
Material and Methods: We analyzed CMR images of AF patients prior to their first catheter ablation. LA fibrosis was assessed using Late Gadolinium Enhancement (LGE) CMR, and LA EAT was assessed using the novel fat-water separation Dixon sequence. Panel A shows a 3D model of the LA depicting LGE fibrosis and superimposed renderings of EAT. Feature tracking was applied to assess global longitudinal strain in its 3 components (reservoir (GLRS), conduit (GLCdS), and contractile (GLCtS)) (Panel B). LA emptying fraction and LA volume were measured using the cine sequences. All CMR images were acquired in sinus rhythm.
Results: 101 AF patients underwent pre-ablation CMR (61% male, average age 62 years). LA volume was 90.4 mL [76.5, 114.35], LA fibrosis percentage was 15.5% [9.6, 19], and LA EAT volume was 28.48 mL [19.14, 34.39]. GLRS was 17.4 [14.95, 20.1], GLCdS was 9.2 [6.75, 12] and GLCtS was 8.1 [6.5, 9.8]. LA fibrosis was negatively associated with the three components of global longitudinal strain (GLRS: R=−0.35, p< 0.001 (Panel C); GLCdS: R=−0.24, p=0.015; GLCtS: R=−0.2, p=0.046). Out of the different sections of the LA, fibrosis in the posterior and lateral walls was most negatively correlated with GLRS (R=−0.32, p=0.001, and R=−0.33, p=0.001, respectively) (Panel D). LA EAT was negatively correlated with GLCdS (R=−0.453, p< 0.001) (Panel E). LA fibrosis was negatively correlated with LA emptying fraction but LA EAT was not (R=−0.27, p=0.007, and R=−0.22, p=0.1, respectively). LA EAT and fibrosis were both positively correlated with LA volume (R=0.38, p=0.003, and R=0.24, p=0.016, respectively).
Conclusions: LA fibrosis and EAT, two important contributors to the AF substrate, are associated with a worsening LA function through strain analysis by CMR. These may play a role in the pathophysiology of thromboembolic stroke associated with AF.
Material and Methods: We analyzed CMR images of AF patients prior to their first catheter ablation. LA fibrosis was assessed using Late Gadolinium Enhancement (LGE) CMR, and LA EAT was assessed using the novel fat-water separation Dixon sequence. Panel A shows a 3D model of the LA depicting LGE fibrosis and superimposed renderings of EAT. Feature tracking was applied to assess global longitudinal strain in its 3 components (reservoir (GLRS), conduit (GLCdS), and contractile (GLCtS)) (Panel B). LA emptying fraction and LA volume were measured using the cine sequences. All CMR images were acquired in sinus rhythm.
Results: 101 AF patients underwent pre-ablation CMR (61% male, average age 62 years). LA volume was 90.4 mL [76.5, 114.35], LA fibrosis percentage was 15.5% [9.6, 19], and LA EAT volume was 28.48 mL [19.14, 34.39]. GLRS was 17.4 [14.95, 20.1], GLCdS was 9.2 [6.75, 12] and GLCtS was 8.1 [6.5, 9.8]. LA fibrosis was negatively associated with the three components of global longitudinal strain (GLRS: R=−0.35, p< 0.001 (Panel C); GLCdS: R=−0.24, p=0.015; GLCtS: R=−0.2, p=0.046). Out of the different sections of the LA, fibrosis in the posterior and lateral walls was most negatively correlated with GLRS (R=−0.32, p=0.001, and R=−0.33, p=0.001, respectively) (Panel D). LA EAT was negatively correlated with GLCdS (R=−0.453, p< 0.001) (Panel E). LA fibrosis was negatively correlated with LA emptying fraction but LA EAT was not (R=−0.27, p=0.007, and R=−0.22, p=0.1, respectively). LA EAT and fibrosis were both positively correlated with LA volume (R=0.38, p=0.003, and R=0.24, p=0.016, respectively).
Conclusions: LA fibrosis and EAT, two important contributors to the AF substrate, are associated with a worsening LA function through strain analysis by CMR. These may play a role in the pathophysiology of thromboembolic stroke associated with AF.
